
Aeson Artificial heart from Carmat. The first biocompatible, biventricular mechanical heart. Carmat has developed a biocompatible, biventricular mechanical heart that is designed to simulate the functionality and morphology of the human heart as closely as possible using self-regulatory mechanisms and biocompatible materials. It aims to provide long term. A solution for patients with advanced biventricular HF and possibly acute myocardial infarction (MI) for whom no human transplant or the possibilities of treatment are available.
The device recently won regulatory approval from European Commission. It now plans to begin sales in the second quarter of this year. Named Aeson, the device is designed to mimic a real heart using biological materials and sensors. Stephane Piat CEO of Carmat says the idea was to create a device that would replace heart transplants and works physiologically like a human heart that is pulsating, self-regulated, and compatible with blood. For now, it has only been approved for those awaiting a heart transplant.
[monsterinsights_popular_posts_inline]
Also Read: Airbus hydrogen fuel aircrafts.

Highlights
- The company is set to launch commercially in Q2 2021, with an initial focus on Germany and France
- A market that can be manipulated at the transplant to transplant sign of more than 2,000 patients a year in Europe.
- Control a clinical plan to support sales adoption and development.
- A virtual conference will be held at CET with Stephane Piat.
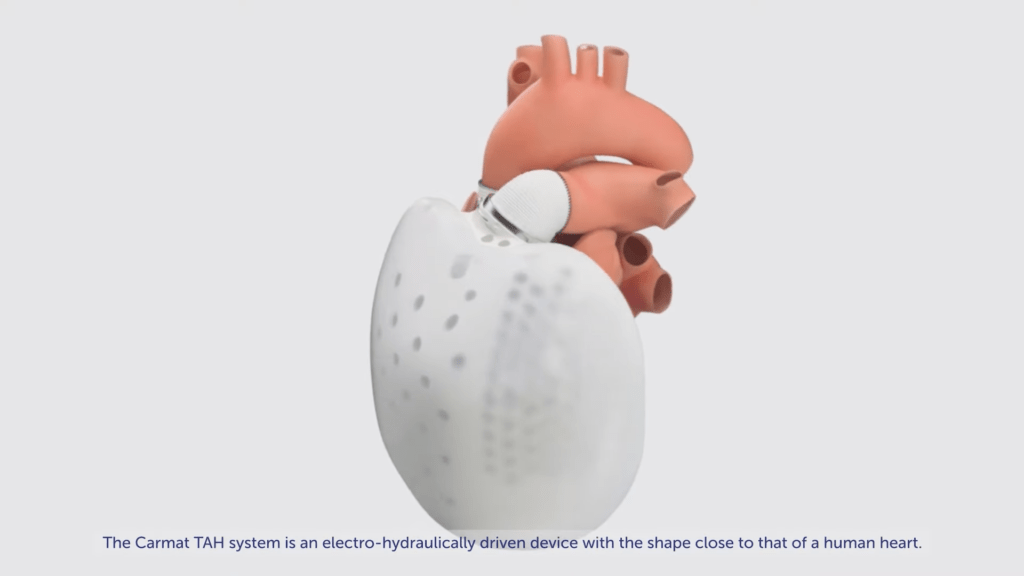
Aeson Artificial heart from Carmat the total artificial heart aims to mimic human function. The first biocompatible, biventricular mechanical heart
Carmat was founded in 2008 as a product of a partnership between Matra Défense (now part of Airbus Group), a technology company, and Professor Alain Carpentier, a cardiologist. bioprosthetic heart, separated from previous generations of artificial hearts to make the prosthesis work as closely as possible to the human heart, using technologies that include self-regulatory methods and incompatible building materials. Carmat’s bioprosthetic heart aims to provide long-term (permanent) treatment, or destination (DT) treatment for patients with advanced biventricular HF or acute MI (commonly referred to as a heart attack) with no human implants available and eliminating all remaining opportunities for treatment.
After completing the fourth patient probability test in early 2016, Carmat started a pivot that allows for a 20-patient mark Trial in July 2016, where registration was suspended in November 2016 after the death of the first hired patient. The study resumed in May 2017 following the cause of death was related to patient battery abuse and in July 2018, the device was successfully installed in patient 10, marking the completion of the first part of the CE empowerment trial. In September 2018, 11 patients underwent surgery with Carmat TAH However, by the end of 2018; product production was suspended following an analysis that identified an area of potential improvement especially in terms of device integrity and technical component cleanliness. Production has commenced and the company expects to resume installation in Q319 So we have delayed our expectation to complete the registration to H120 (earlier in mid-2019) and launch in Europe by the end of 2020 (before H120) to m find an important clinical need for alternative treatment permanent patients on waiting lists for heart transplants. The lack of any such bilateral implant for permanent acceptance (DT) in either the EU or the US can be a significant commercial advantage.
Also Read: Mayflower Autonomous Ship (MAS) from ProMare and IBM
CARMAT (FR0010907956, ALCAR), a world-class artificial heart developer and engineer, which aims to meet the unmet medical need by providing alternative therapies for people suffering from biventricular heart disease, today describes the commercial and development system with all its artificial heart. A virtual conference was held on January 6th, 2021 to outline the commercial and development plan for Aeson Artificial heart from Carmat.
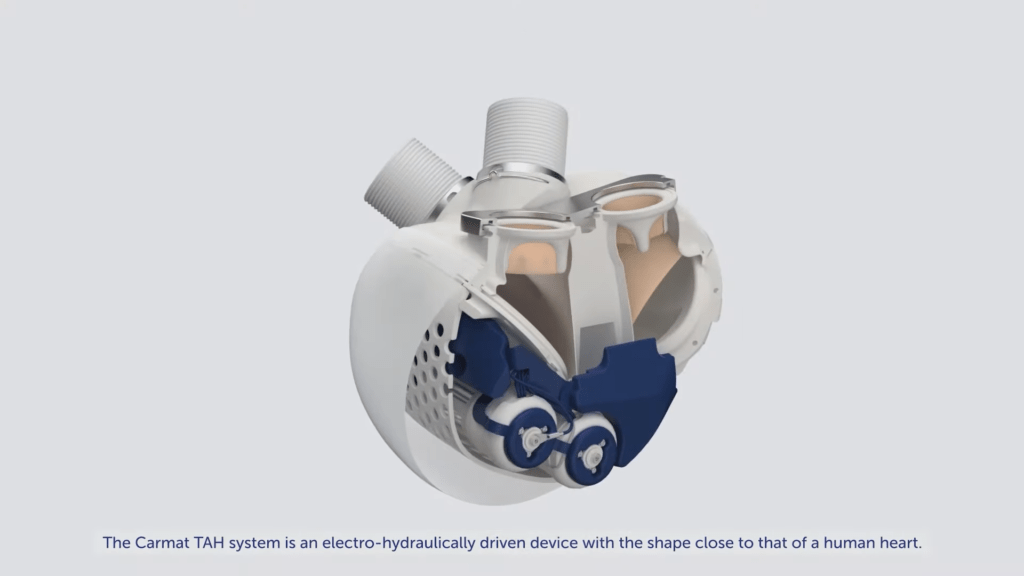
In 2020, despite the COVID-19 situation, CARMAT has implemented key goals including:
- Gaining full approval from the US Food & Drug Administration (FDA) to arrest Early Feasibility Study (EFS) in the United States and successfully trained three US centers in Q4 2020 to initiate transplantation in Q1 2021.
- Secured 13 million Euros from the French government to start a new clinical trial (the “EFICAS study”) in France in the second quarter of 2021.
- Received a CE Mark which allows CARMAT to market its total number of artificial hearts as a bridge for transplantation in a large number of countries, including all of the European Union.
- Continued to study PIVOTAL in France, with 2 patients being treated in December.
Before receiving the CE mark, CARMAT had taken the necessary steps to be ready to begin selling its artificial heart from Q2 2021. This includes:
- Proactively speeding up the ramping up of manufacturing activities.
- Targeting customers and early hospital support for a refund.
- Product positioning and branding.
Aeson artificial heart from Carmat will be marketed under the brand name Aeson. Based on 3 different product features – pulsatility, autoregulation2, and hemocompatibility – CARMAT creates a new product category: Physiologic Heart Replacement Therapy (PHRT).
The CE marking of CARMAT’s heart as a transplant bridge (BTT) indicates a significant market opportunity with fewer than 2000 patients currently on the waiting list for heart transplants in five major European countries.

In 2021, the Company plans to focus on Germany and France, which together account for 55% of the mechanical circulation assistance (MCS) market in the European Union:
- Commercially Aeson launched in Germany in Q2 2021.
- The French market is initially addressed through the EFICAS study.
Besides, the company could seize further sales opportunities in other countries recognizing the CE mark.
Similarly, CARMAT will continue to implement a more robust clinical program that includes the EFICAS Study in France (52 patients), the completion of an ongoing PIVOTAL study (goal of 20 patients), and follow-up of a major post-sales clinic, which will involve the first 95 patients treated in a commercial setting, to produce more safety, performance, and health economics data in the product (DT) of the product. When the BTT signal allows for temporary support of the device, this additional DT signal will allow CARMAT to identify patients who are not eligible for heart transplants who will remain under CARMAT device support in the long run. CARMAT ensures that its available resources enable the company to fund its operations up to Q3 2021. It considers various options to fund its future development.
The chief executive officer of CARMAT, Stephane Piat says that “2020 is a decisive year for the company and we got the CE marking just before Christmas. Despite the very difficult environment marked by COVID-19, CARMAT has achieved most of its purpose. Thanks again to the entire CARMAT team and all the stakeholders who made this possible. The signs of a bridge to transplantation obtained as part of the CE marking are patient hope and at least 2,000 people. It represents a huge market opportunity for the company. Patients are currently on the waiting list for heart transplants in Europe, but very few are lucky enough to benefit from donor transplants. 2021 Will focus on Germany and France, which account for more than half of the total, and may respond in a more optimistic way to other countries that allow CE marking. In the second quarter of 2021, we are pleased to confirm that the product is ready to go on sale.

In addition to carrying out our strong clinical plan, especially the EFICAS study, which will begin in the second quarter of 2021, 95 people We will also perform post-marketing clinical follow-up for transplant patients, which will have a significant impact on the momentum of our prosthesis adoption. Aeson, a unique feature demonstrated in previous clinical trials, is terminally ill. Introducing Physiological Cardiac Replacement Therapy, a new way to treat heart failure, this aims to significantly improve the quality of life of patients.
- CE marking is a bridge-to-transplant (BTT) for patients with end-stage biventricular heart failure that cannot reach a maximum on CARMAT’s entire artificial heart system on December 22, 2020, has been granted. Medical therapy or LVAD (Left Ventricular Assist Device) and people who are likely to receive a heart transplant within 180 days after transplantation.
- The “PHRT” category was created by CARMAT and distinguishes the “TAH” (Total Artificial Heart) category, which is a unique combination of three features: pulsation, autoregulation, and blood compatibility.
- The initial inclusion target for this study was 20 patients, a figure that can be adjusted up or down during the study. To date, in the study, 15 patients have been enrolled.


